We will try to describe in some depth the electrical system of a conventional car, micro-hybrids, hybrids and electric cars. But before we get into the subject, we are going to explain in a summarized way some concepts that are necessary for the understanding of the electrical system. We will omit the sensors, relays, fuses, and other accessories to not make it confusing. If you already know these concepts, you can move on to the next point. And those who don't, I will try to be as didactic as possible.
Previous concepts
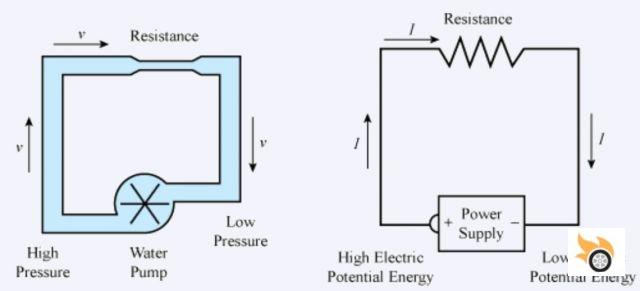
As we were saying, we are going to see some things that we are all familiar with, but that we may not know in depth. To do this, we will make an analogy with a hydraulic system, more intuitive and easier to understand.
- Current: Known in electricity by the symbol "A", for ampere. It basically measures the flow (amount per unit of time) of electrons passing through a wire. Analogously to a pipe, which would be the flow in liters per second. The higher the current, the thicker the wire must be. As in a pipe, the greater the flow, the greater the diameter required.
- Voltage: Its symbol is "V", from volt. It is a measure of electrical potential, and the analogy in hydraulics would be the pressure in a pipe. The higher the pressure, the more current there can be (that is why it is potential). It is like a closed tap (switch): it is holding the pressure (tension) of the pipe (cable), but there is no flow (current). But if it is opened, we have flow (current).
- Resistance: Resistance is known by the symbol "R", and is measured in ohms, in honor of Mr. Georg Simon Ohm, who listed a very important law for electricity, which we will see later. Continuing with the electrical analogy, it would be a constriction in our pipe. This helps to modify the flow (current) we have in the circuit. Although at the cost of losing pressure (voltage) that is dissipated as heat.
- Capacitor: The capacitor has the symbol "C" and is measured in farads, in honor of Michael Faraday. In electricity it serves two purposes: placed in series, it blocks direct current and lets alternating current through. And in parallel it would be like a small current store: it stores it and lets it out when necessary. In hydraulic analogy it could be likened to an intermediate tank, to maintain the flow in the pipe.

- Ohm's Law: Ohm's law relates voltage, current and resistance. It simply states that: V = I * R. Thus, we can calculate resistance, if we know voltage and current: R = V / I, or the current if we know voltage and resistance: I = V / R.
- Electrical power: Electrical power is measured in watts and has the symbol "w". It is calculated as: P = V * I. Using Ohm's law, it can also be expressed as: P = V² / R or P = I² * R.
- DC voltage/current: The DC voltage or current is the one that comes from the batteries. It is always constant, it does not vary between positive and negative. In English it is known as DC (direct current).
- Alternating voltage/current: This is the voltage or current that we have in the sockets at home. This voltage/current oscillates from positive to negative 50 times per second (50 Hz). That is, it varies from +220 V to -220 V at 50 Hz. In English it is known as AC (alternate current).
- High voltage: For our case in the car, it will be when we have the order of hundreds of volts (100 to 600 V normally). We will know it as HV. In the case of the design of distribution networks or power generation, the voltage is much higher, in the order of thousands of volts.
- Low voltage: Inside the car will be the network of 12 V or 48 V, in older models was 6 V. We will know it as LV
The concepts are quite heavy, but necessary to understand how a battery behaves. It is something similar to the pieces of a puzzle, now it will look messy, but at the end of the article the pieces will be ordered and will make sense. Let's take a look at the most important parameters of the two fundamental components: the battery and the electric motor/generator.

The battery
Now we are going to talk about the batteries, fundamental in any electrical system of any vehicle, and of greater relevance now that the electrification of cars is coming. Knowing how they work, we will understand much better the concepts applied to hybrid and electric vehicles.
Cell and battery
If you have read our colleague Dinesh's article you will have seen a very detailed explanation of how a lead-acid cell works. Wait, what is a cell? Take a look at the picture at the top of this part: it's a naked Tesla Model S. If you look closely, you can see that there are hundreds of cylindrical cells inside. For experts in the field is the minimum unit that performs the function of generating electricity from a chemical reaction. Basically it is a production standard. A battery will be composed of two or more cells in series (usually) to achieve the desired voltage. In the case of an automotive battery, each lead-acid cell produces on the order of 2 V nominal. Therefore, a car battery has 6 cells in series. That is, 6S. What does 6S mean? Let's look at it.
Types of batteries
Now that we know the difference between a cell and a battery, let's see what types of batteries there are, depending on the configuration of the cells. Those of you who like model airplanes will know this very well. Since a battery is a grouping of cells, depending on how they are placed, we will get a different battery, depending on what we want to achieve. If we place them in series, it is indicated with "S", and if in parallel, with "P".
Let's take an example. Let's imagine that we have 4 LiFePo4 cells of 3.2 V and 1 Ah. The four configurations that we can obtain are:
- 4S: The 4 cells in series. We will have the sum of the voltages, but the same current. Therefore the 4S battery will be 12.8 V and 1 Ah.
- 4P: The 4 cells in parallel. We will have the same voltage, but the sum of the currents. The resulting battery 4P will be 3.2 V and 4 Ah.
- 2S2P: First we place two cells in series (6.4 V and 1 Ah) and then we put them in parallel with two others. The resulting battery would be 6.4 V and 2 Ah.
- 2P2S: If we place two cells in parallel (3.2 V and 2 Ah) and connect them in series with another equal block, we obtain a battery of 6.4 V and 2 Ah. Same result as 2S2P, but with a subtle difference. In this case all the cells are connected, balancing the currents better.
In short: if we need more voltage, we add cells in series. If we need more current, we add cells in parallel.
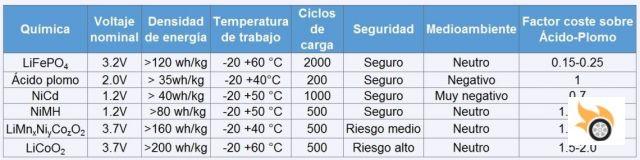
The chemistry of electricity
Depending on what kind of chemical compounds are used inside the cell, we will obtain specific characteristics in energy density, charge/discharge cycles, voltage, current, etc. I attach a summary table so you can see the differences at a glance.
For what we are interested in, NiMH are those of Nickel Metal Hydride (as used by Toyota in the Prius). The ones with this unpronounceable name, LiMnxNiyCozO2, are Lithium Ion, like the ones we have in our mobile phones. Some variation in the compound is often used, such as Tesla, which uses LiNiCoAlO2. But generically they are called Lithium Ion. And finally, the lead acid, which we carry in the car. A note: AGM or gel batteries have slightly different voltages than lead-acid batteries.
And why have I written this "tocho"? Well, precisely to understand the following point, which is key in the electrical system. How energy is stored in the battery.
Charging and discharging voltage
Depending on the voltage that we see in the table cells according to their chemistry, and within a few margins, a cell will be charged or discharged. Let's take the example of the lead-acid cell. If its voltage (or voltage) reaches 2.4 V, it is charged. And if it has 1.9 V, it is discharged. If we multiply this voltage by the cells in series that we have (remember 6S), it gives us a limit of 14.4V for charging and 11.4 V for complete discharge approximately. Beyond these values, we will be destroying the battery.
Each type of cell, will have some values recommended by the manufacturer, charge and discharge. In general, they should not be charged more than 100% (exceeding the maximum charge voltage), and discharged below 20%. In the case of lead-acid, 20% discharge would be just at 12 V. By maintaining these safety margins, we guarantee that we will not destroy the battery throughout its charge and discharge cycles. It is for this reason, that even if a manufacturer advertises a certain capacity in its battery, the usable margin is less. The battery will never be below 20%. And usually, it will not reach 100% either, to leave room for regenerative braking recharging. Therefore, when you see a certain battery capacity, stick with 70%, which is what is really usable.
How fast can I charge and discharge a battery? Well, it depends on its chemistry. The manufacturer of the cell gives us the recommended value in charge and discharge in factor "C". For example, we have a LiFePo4 cell, with 3.2 V nominal, 20 Ah (amps per hour), 2 V as maximum discharge point, and 3.7 V as maximum charge, and tells us to use 1 C in charge and 5 C in discharge. Well, simply multiplying by the amount of Ah that the battery has. In this case, we can charge the cell with 20 A, and discharge 20 x 5 = 100 A.
Power
What is the power of this cell? Well, if we multiply voltage by current, we have 64 W in charge and 320 W in discharge.
Let's see now a car battery, and how to interpret the data. For example, a 12 V, 60 Ah and 680 CCA (Cold Cranking Amps). The CCA would be the maximum discharge capacity of the battery, and if we divide 680 by 60, we see that it is approximately 11 C. The rated power of the battery would be 12 x 60 = 720W. The maximum power would be 12 x 680 = 8,160 W.
Let's explain what Ah means. Amps per hour gives us an idea of the capacity of the battery. It means that if we use 60 amps of discharge, the battery will be drained in one hour. If we use 30 amps, it will last two hours. And so on.
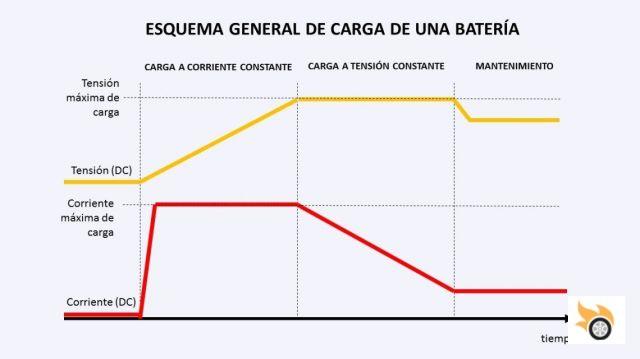
Charging the cell
So how do we charge it? This is where the charging curve comes into play. There are generally three phases to charging: charging at constant voltage, charging at constant current, and maintenance. In the first phase, the current is left constant, and the battery rises in voltage. When it reaches its maximum voltage, the voltage is left constant, and the battery will draw less current over time. When the current approaches zero, the battery is fully charged. From then on, we will lower the voltage a little, and leave a small maintenance current.
Therefore, the more current a cell provides, the faster it can be charged and the more power output it will have. This is what our mobile phone does every day, when we connect it to the charger.
Life of a cell
Let's go now to the life of the cells. Apart from respecting the maximum and minimum voltage, it is important to keep them within their working temperature. Batteries also have resistance, so when they are charged and discharged, they get hot. They do not like excessive cold or excessive heat. Their ideal temperature? Like ours: about 25 ºC, with 5 ºC up or down. At that temperature they work well and will last their whole life without any problems. At a higher temperature, it will offer a little more performance, at the cost of battery wear and tear. At lower temperatures, performance drops rapidly. Therefore, it is necessary to control their temperature for good performance.
Why do they wear out? Quickly explained: with charging and discharging, the electrodes expand and contract, producing over time micro-cracks that cause the cell's performance to decline. The number of cycles we saw in the previous table guarantees that, after that number, the cell maintains 80% of its capacity. Therefore, over time, the cell will store less energy. That's why the battery of our mobile lasts less, the more time it has. Remember that these cycles are for full charge, that is, when you reach 0% and charge it to 100%. Therefore, if you recharge it twice from 50% to 100%, it will count as a single cycle.
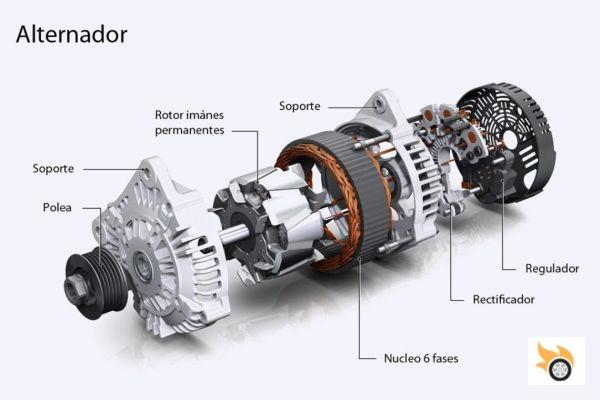
The electric motor/generator
An electric generator is a device that transforms motion into electricity. An electric motor transforms electricity into motion. It's as simple as that. There are different types, depending on their design. But they have in common that there is always a moving part (rotor) and a fixed part (stator).
Generally, there is reciprocity in electric motors: if you move them, they generate electricity. And also in generators: if you apply electricity to them, they move. Therefore, they are generally known as electric machines, because fundamentally a motor and a generator are the same thing, just used in a different way. Whether they can be reciprocating depends on their design, mainly the associated electronics.
As an example, let's use the car alternator. Its function is to generate electricity, and initially, it does not work as a motor. With some small modifications in its electronics, it is possible to use it as an electric motor. To simplify, we will classify the motors/generators in two types: those that work/generate direct current (DC) - Does anyone remember the Scalextric cars - and those that work/generate alternating current (AC). Continuing with the example of the alternator of the car, it generates alternating current. But it converts it into direct current by means of electronics.
Well, we are done with the boring part of the theory, and we have laid the groundwork for understanding how the electrical systems of any car are designed. In the next part we will see the differences between them...